SARS-CoV-2 was transmitted as a zoonotic infection from animals to humans. It crossed the species barrier that normally prevents pathogen transfer. A virus is well adapted to its host organism and thus is depending on the specific conditions present in this organism. Therefore, crossing the species barrier may well lead into a cul-de-sac. In such a case of a dead-end infection, the virus is unable to spread.
In rare cases, random mutations lead to adaptation of the virus to its new host. This results in an evolutionary development of the viral population. Those viruses with a selective advantage within the host organism will have a higher multiplication rate. In order to spread within a human population, person-to-person transmission needs to be efficient.
The virus is dependent on the presence of a host organism. Consequently, once a virus is not able to infect a new host the chain of infections ends. The virus disappears.
SARS-CoV-2 adapted to the human organism in a way that it is able to spread person-to-person.
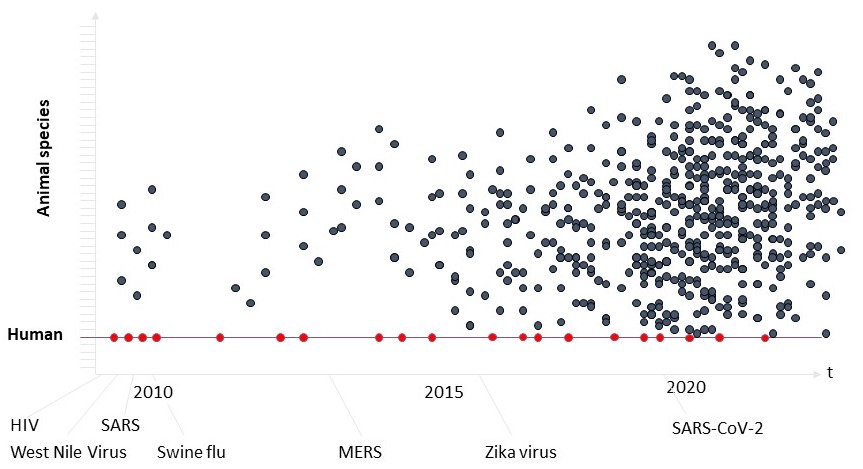
Zoonoses happened in the past. Human immunodeficiency virus (HIV) is a notorious example. More than a hundred years ago it was transmitted from apes and monkeys to humans. Most likely this happened during hunting of apes and monkeys and the slaughtering for bushmeat. Slaughtered animals carried a virus (simian immunodeficiency virus, SIV). Infections by this virus coming from chimpanzees and sooty mangabeys resulted in the currently known HIV-1 and HIV-2. Another prominent example is influenza. Influenza viruses are circulating between pigs, poultry and humans, resulting in new variants of influenza on a regular level. Further examples would be Ebola virus, Rabies virus and Hanta virus, that are transmitted to humans from many animal species.

What happens next? The better the virus is adapted to the new organism, the better it will be spread. In that situation, lower morbidity and mortality is expected. Should a virus weaken or kill its organism too fast, it will not spread as far as a variant with weaker symptoms or a prolonged course of disease. This circumstance leads to a selective pressure onto the viral population.
What do we have to expect?
Scenario A: The virus is able to spread with low efficiency. Once it cannot infect a new host organism it will disappear from the population. The SARS epidemic of 2002/03 is one example. The virus was not able to spread very fast and could be contained. Another example would be smallpox: For thousands of years the Variola virus circulated in the human population. The global vaccination campaign severely reduced the number of susceptible people, the virus could no longer spread efficiently and finally disappeared from the human population.
Scenario B: The virus is adapting in a way that mortality and morbidity are low enough for it to establish an ongoing infection of the human population. It is here to stay. Examples include rhinoviruses (the common cold) and herpesviruses. Herpes accompanied us and our ancestors for millions of years, it is an “heirloom virus”.
This may even go as far as a virus infecting the germline of its host, thereby becoming a part of its host. This, of course, is an evolutionary process taking millions of years. Mammalians, which we are part of, are carrying the remnant of a retrovirus. This protein is responsible for the formation of the syncytiotrophoblast within the uterus during pregnancy. Without this remnant we wouldn’t be mammalians. This, of course, happens in huge time scales. What do we have to expect in the coming months with SARS-CoV-2?
There are already a number of scientific publications regarding SARS-CoV-2. One cellular receptor has been identified to bind SARS-CoV-2. This receptor is found on several cell types and tissues, enabling the virus to bind to these cells/tissues. Thus, different kinds of tissue are susceptible to the virus.
The good news is: Now scientists are able to test drugs that have been developed after the SARS epidemic of 2002/03. While SARS-CoV-2 seems to be more infectious, the general molecular mechanism seems to be similar to SARS-CoV (of 2002/03). Now it is possible to develop therapeutic assays.
However: Viruses do have the last word. The animal kingdom is brimming with unknown viruses. Now the time has come for the development of prophylactic and broadly acting antiviral molecules. This is our area of expertise. This is what sajo is offering.

Stay tuned,
Sabine and Joerg


